Delayed: Explaining one of Big Pharma’s favorite tricks
News | June 1, 2018
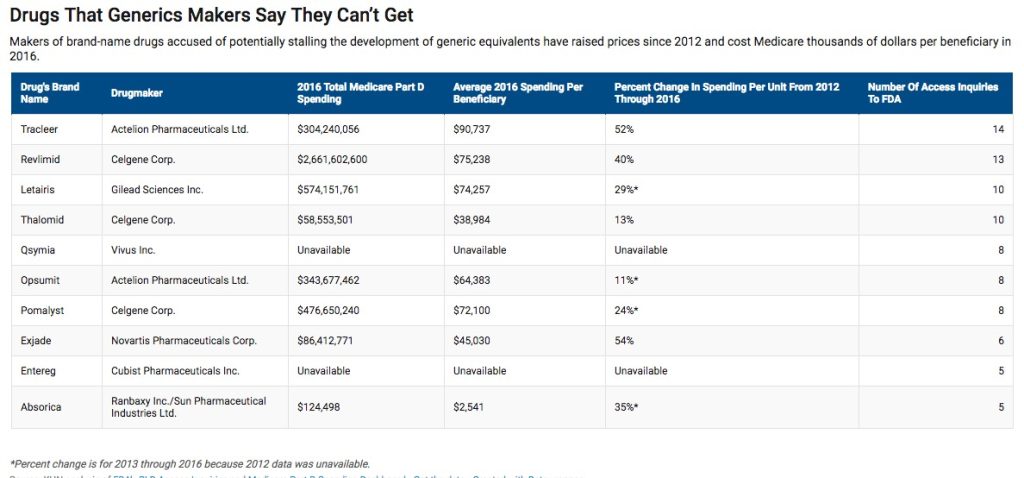
In an attempt to encourage and improve transparency, the FDA released a list of more than 50 drug makers accused by generic drug corporations of stalling providing samples that would increase competition and lower prices.
Typically, when a company plans to bring a generic drug to market, it must first show that its drug is exactly that — a chemically similar product. According to the FDA, instead of providing generic companies with the needed sample, drugmakers have employed “gaming tactics” in which “potential generic applicants are prevented from obtaining samples of certain brand products necessary to support approval of a generic drug. Generic drugmakers’ inability to access these samples impedes, and even many times, completely stops the process of getting a generic drug to market.”
According to Kaiser Health News, the drug makers on the FDA’s list are also responsible for double-digit percentage price hikes since 2012, which cost Medicare and Medicaid nearly $12 billion in 2016.
Celgene Corp. is at the top of the list, with over $2.6 billion in Medicare Part D spending for its blockbuster cancer drug, Revlimid. The company also took first place with a whopping total of 31 inquires for access to generic samples — 14 of those requests for Revlimid.
Read more here at KHN.